While sodium is cheaper than lithium, and sodium ion cells can theoretically store plenty of energy, potential electrode materials are not proving equal to the challenge of storing sufficient sodium ions without being broken by the movement of such large atoms – graphite is thought a non-starter, for example.
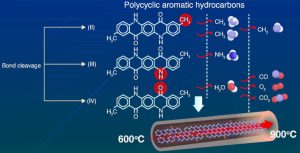
The Pusan anodes are carbon-containing (‘carbonaceous’) with desirable attributes such as well-spaced disorganised carbon and certain helper atoms, made by heat-modifying (‘pyrolysing’) carefully chosen starter materials – specific quinacridones in this case, which are dyes.
“Organic pigments such as quinacridones have a variety of structures and functional groups,” said Pusan professor Seung Geol Lee. “As a result, they develop different thermal decomposition behaviours and microstructures. When used as a precursor for energy storage materials, pyrolysed quinacridones can greatly vary the performance of secondary batteries. Therefore, it is possible to implement a highly efficient battery by controlling the structure of organic pigments precursor.”
![]() The team picked 2,9-DMQA (2,9-dimethylquinacridone), which has molecules that stack parallel when fresh, and keeps a 3D structure that is accessible to sodium ions when pyrolised. Plain unsubstituted quinacridone in contrast clumps during heating.
The team picked 2,9-DMQA (2,9-dimethylquinacridone), which has molecules that stack parallel when fresh, and keeps a 3D structure that is accessible to sodium ions when pyrolised. Plain unsubstituted quinacridone in contrast clumps during heating.
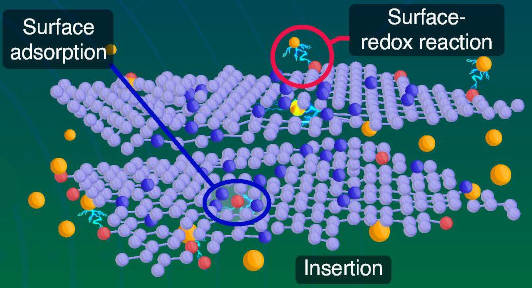
As well as being accessible, heating produces the high disordered carbon content, and nitrogen and oxygen groups that improve ion accommodation through insertion into graphitic layers, said the team, while promoting surface redox reactions and surface adsorption – allow it to store 290mAh/g at 5mA/g, 247 mAh/g at 100mA/g after 200 cycles, and 134mAh/g at 5A/g for 1,000 cycles.
The work is published as ‘Longitudinally grown pyrolyzed quinacridones for sodium-ion battery anode‘ in Chemical Engineering Journal – only a restricted version is available without payment.